Tourette’s
syndrome:
An examination of dominant discourses and power relations in the genetic controversy
Presented to
Faculty of
Simon Fraser University
_____________________
In Partial Fulfillment
of the requirements for
Master of Arts
in
Liberal Studies
____________________
by
Miguel B. Llora
Spring 1998
Tourette’s
syndrome:
An examination of dominant discourses and power relations in the genetic controversy
1.0 Introduction
In the tradition of the 19th century classification debate surrounding Tourette’s syndrome (TS), history is repeating itself in the controversy surrounding the unorthodox genetic theories of Dr. David Comings. Drawing from his years of treating patients, Comings has come to theorize that TS is more than a tic disorder. Much like his predecessor Guinon, Comings also asserts that his colleagues have too narrowly defined their disorder. Comings’ genetic studies have led him to conclude that TS is a polygenic disorder, caused by the convergence from both parents of several genes which affect the levels of dopamine, serotonin and other brain chemicals.
Because Comings believes that TS is genetic, he speculates that a gene, which is semi-dominant and semi-recessive, is involved in TS. Moreover, he has defined TS as a spectrum disorder, which includes a host of other behavioral disorders such as alcoholism, drug abuse and compulsive eating disorders. He feels that by defining TS as an autosomal dominant condition, his colleagues’ efforts are doomed to fail. This theory has dramatic implications regarding the causes of certain behaviors that mental health professionals have traditionally attributed to psychological problems, poor parenting or learning behaviors.
Comings represents the most thorough
and persistent view of the genetic component of TS. In this light, Comings’
views are worth understanding and must be taken seriously. However, he is
not without opposition and his findings have undergone extreme scrutiny and
criticism. Ranging from the absurd to the technical, Comings has been accused
of conjuring up the ghosts of Eugenics to merely being guilty of an ascertainment
error. His perspective and the objections of his detractors are the subject
of this work. In the end, whether Comings is ultimately vindicated is moot.
What is important is, as we inch closer to a full explanation of the etiology
of TS, a possible explanation has not been ignored by the reductionist tendency
of specialization.
2.0 What is To urette’s
syndrome?
With regards to the genetic dimension of TS, the phenotype observation determines the etiology. Since there is no agreement on the phenotype, there is no agreement on definition, prevalence or intervention. In order to understand the phenotype differences, it is essential to begin with the most basic definition. A phenotype is linked by definition to a genotype. A genotype can be defined as the genetic constitution of an individual or group, or as a class or group of individuals sharing a specific genetic makeup. In this light, a phenotype is the visible properties of an organism that are produced by the interaction of the genotype and the environment. More importantly, it is a group of organisms sharing a particular phenotype. The phenotype standard for the studies examined in this work use the diagnostic criteria from the Diagnostic and Statistical Manual 3rd Edition-Revised (DSM III R) as its base. It is essential for this discussion to outline the DSM III R as it will continuously come under fire as the efforts to develop a standard are undertaken. The DSM III R definition is outlined below:
307.23 Tourette’s Disorder
A. Both multiple motor and one or more vocal tics and have been present at some time during the illness, although not necessarily concurrently.
B. The tics occur many times a day (usually in bouts), nearly every day or intermittently throughout a period of more than one year.
C. The anatomic location, number, frequency, complexity, and severity of the tics change over time.
D. Onset before age 21.
E. Occurrence not exclusively during Psychoactive Substance Intoxication or known central nervous system disease, such as Huntington’s chorea and postviral encephalitis.
3.0 Statistics on Tourette’s
syndrome
In a prevalence study done by Anne Mason, Sube Banerjee, Valsamma Eapen, Harry Zeitlen and Mary M. Robertson; five DSM III R cases of GTS were identified out of 167 pupils yielding a prevalence estimate of 299 per 10,000. This estimate leaves the prevalence estimate close to a significant 3%. The DSM IV prevalence estimate (based on the DSM IV phenotype) is 4-5 individuals per 10,000. In the same study, four previous prevalence studies were identified and compared. Figures have come from highly selected tertiary care centers.
There are three reasons why they feel that the information is not free from selection and attribution bias: 1. Complex filters are used to get access to tertiary care. 2. Recognition and diagnosis of GTS is uneven. 3. Referral may be dictated by associated behavioral disturbance. Building on this argument, Robertson et al make a comparison of four previous studies, producing a marked difference in the results.
Burd, et al (1986) came up with .50 per 10,000 (0.77 per 10,000 for males; 0.22 per 10,000 for females). Caine, et al (1988) - 2.9 per 10,000. This represents a three-fold increase from Burd, et al (1986). Comings, Himes and Comings (1990) - 105.2 per 10,000 for males and 13.2 per 10,000 for females of school age, 75.8 per 10,000 overall. 75.8 per 10,000 is a considerable 25 fold increase from Caine, et al (1988) estimates. Comings represents the widest phenotype examination. The details of which are the central focus of this section. Apter, et al (1993) - 4.2 per 10,000 (4.9 per 10,000 for males; 3.1 per 10,000 for females).
In this light, their contention that no study to date has carried out a systematic examination of a whole school age population is worth noting. Moreover, they identify “recognition and diagnosis of TS is uneven” as a second reason for an inherent bias. If for prevalence accuracy alone, the need for a standard is considered vital.
The main finding of this study was the strikingly high prevalence of GTS. They identified five DSM III R cases of GTS in the single school studied. This yields a prevalence estimate of 3%, a rate four times greater than Comings et al, 1990, the next highest reported. If the clinic population were used to calculate prevalence, then the rate would be 4.8 per 10,000; this clearly illustrates the bias inherent in the use of clinic samples. 4.8 as opposed to 299 would make 1 in 60 cases reported.
4.0 Genetic Models
4.1
Pauls, et al - 1992 -The Autosomal Dominant Inheritance Model:
Pauls, et al - 1992 advocate an Autosomal Dominant Inheritance Model. In line with the DSM III R, they endorse a narrow spectrum whose phenotype includes only tics and OCD. They hypothesize that the genetic linkage of the TS Spectrum is inherited as a single gene. Their model is one of autosomal dominant inheritance, with variable expression and reduced penetrance (Comings, Tourette Syndrome and Human Behavior 41).
This form of inheritance is called autosomal because the gene is on one of the autosomal chromosomes, as opposed to a sex chromosome. It is dominant because only one abnormal gene is required to produce the disease; that is, the abnormal gene is dominant over the normal gene. In this type of inheritance there is a 50 percent chance that any child will inherit the abnormal gene, and males and females are equally affected. Multiple generations are often affected in genetic disorders that are inherited in an autosomal dominant fashion (Comings, Tourette Syndrome and Human Behavior 41).
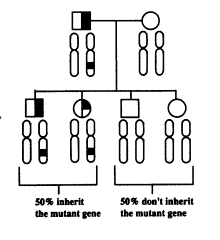
In order to produce the statistics for the segregation analysis, a family study is done. The graphic representation of the data collected results in a pedigree study. The explanation is taken from Mark Kozlowski’s home page. Mark is a TS patient and has done his own pedigree.
4.1.1 Pedigree Studies:
(As outlined in the Tourette’s Syndrome Home Page of
Mark Kozlowski)
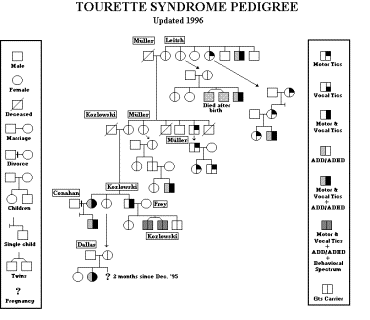
A TOURETTE SYNDROME PEDIGREE — How to construct one:
What is a pedigree? A pedigree is simply a diagrammatic presentation of a family history. It is a tool that researchers use to gain a better and more macroscopic view of an individual’s family gene pool. A pedigree does not have to be just a tool for the researcher; it can also be a useful tool for you and your doctor. Recording a beneficial pedigree is a critical part in the evaluation of a patient with Tourette syndrome and the behavioral disorders manifested within it’s spectrum. Too often doctors are simplistic in recording family history. They ask a simple question — “Does anyone else in your family have TS?” Thus, your family history is nothing more than scratch notes and not helpful what so ever.
How do you take a pedigree? First, find out about your mother’s side of the family, i.e. who’s who. Once you have written down the names, ages and gender of all of your mother’s relatives (living or dead), then you can proceed to ask detailed questions about them concerning disorders they had. You are going to have to ask or write to these relatives to get as many details as possible. One source is not sufficient. Second, work on the father’s side of the family and do the same. It is imperative that you be very thorough and ask people again and again as time goes on. A well-done pedigree does take time and sometimes even years.
There are different approaches to taking a pedigree. The first approach would be a very detailed and complex pedigree. This means that everything is included even disorders not connected with TS such as Diabetes for example. This is probably the most pain staking. The second approach is a more moderate type of pedigree. This would include Tic Disorder, ADHD, OCD, Enuresis, Conduct disorder, Alcoholism, and the like. This means that you would be recording disorders individually within the TS spectrum. The third approach is a simplistic one which only records the Tic Disorder, ADD/ADHD, and the behavioral spectrum grouped as one.
I personally took the third approach. I have included my own personal pedigree as an example. Study the use of the symbols I have used. These symbols are a standard tool used by researchers but can be manipulated for the more extensive pedigree’s. For example, the box, which represents the male, can have an ‘A’ in the center if alcoholism is a concern for the pedigree or polka dots could be used to shade the box to represent autism. Nonetheless, the symbols as I have them should be kept the same and only the inside of the boxes or circles should be altered to represent different disorders [no standards would cause confusion]. Additional information can, of course, be written down within the content of the pedigree.
EXAMPLE BELOW:
4.1.2
Segregation Analysis (Comings, Tourette Syndrome
and Human Behavior 48-9):
The difficulty of determining the precise mode of inheritance in TS from single or a few selected families can be appreciated by looking at a few pedigrees. How do we sort all these pedigrees out to determine the real mode of inheritance? This is done by a procedure called segregation analysis in which data from a large number of non-selected or minimally selected cases are examined by various computer programs. POINTER was called into use in a study by Pauls and Leckman of 30 families. The unique aspect of this study was that many of the relatives were individually interviewed, rather than relying on the information from one or two members of the family, and obsessive-compulsive behaviors were included, in addition to motor and vocal tics. The following penetrances were obtained:
|
|
males |
females |
|
Tourette
syndrome |
45% |
17% |
|
TS
or Chronic Motor Tic (CMT) |
99% |
56% |
|
TS,
CMT or Obsessive Compulsive Behavior (OCB) |
100% |
71% |
4.1.3
Penetrance:
The marked increase in penetrance for females is observed when obsessive-compulsive behaviors were included. As indicated by the chart above, the 15% of females carrying the GTS gene expressed penetrance manifestation as OCB. As opposed to the recessive inheritance model, where two alleles are required, the autosomal dominance requires only one is present. Penetrance figures are indicative of the manifestation of the disorder. The 15% of females that manifested OCBs as opposed to the 1% difference for males with OCB is a classic example of variable expression. Non-penetrance means that a person can carry a gene but not show any signs of it (Comings, Tourette Syndrome and Human Behavior 42).
4.2
Comings, et al - 1992 - Spectrum Disorder Model:
In contrast to Pauls, et al, Comings speculate that a gene, which is semi-dominant and semi-recessive, is involved in TS. Comings has defined TS as a broad-spectrum disorder which includes a host of other behavioral disorders such as alcoholism, drug abuse and compulsive eating disorders. He feels that by defining TS as an autosomal dominant condition, his colleagues’ efforts are doomed to fail. A simple reading of Comings, et al, is one of a Serotonin - Dopamine Axis in which defects in serotonin metabolism both cause and complement hyperactivity of dopamine or dopamine receptors (Comings, Tourette Syndrome and Human Behavior 196).
Serotonin - Dopamine Axis (Comings, Tourette
Syndrome and Human Behavior 176).
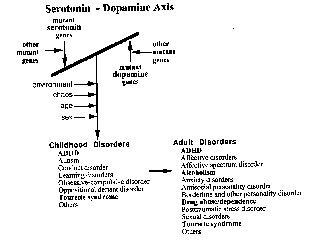
Comings
speculates that a TDO2 gene mutation results in a defective serotonin metabolism.
A number of other loci affecting dopamine metabolism, such as dopamine D2
Receptors allele, can act as a modifying gene. His chemical explanation includes
serotonin, dopamine and norepinephrine. The chemical interaction affects specific
areas of interest, namely, the frontal lobe, the striatum, the hypothalamus
and the limbic system. The frontal lobe is the primary motor area that allows
us to consciously move our skeletal muscles. Specifically this function is
located anterior to the central fissures in the frontal lobe. The striatum
is involved in the control of complex motor activities. The hypothalamus is
an important autonomic nervous system center because it plays a role in the
regulation of body temperature, water balance and metabolism. It is the center
of many drives and emotions. It regulates the pituitary gland and produces
2 hormones of its own. The limbic system is the “Emotional Visceral Brain”.
The hypothalamus controls the limbic system.
|
Dopamine |
Norepinephrine |
Serotonin |
|
Caudate |
Hypothalamus |
Hypothalamus |
|
Putamen (striatum) |
|
Limbic System |
|
Frontal Lobe |
|
Frontal Lobe |
4.2.1
Serotonin:
TDO2 (Serotonin)
(Comings, Search for the Tourette Syndrome and Human
Behavior Genes 50).
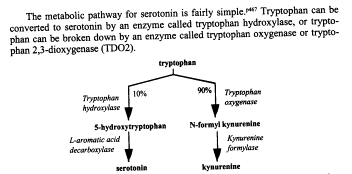
4.2.2 Dopamine:
Comings concentrates on three dopamine nerve pathways. [1.] The nigro-striatal pathway, [2.] The ventral tegmental area, and [3.] The tuberoinfundibular pathway. The substantia nigra-caudate-putamen (striatum) are included in the nigro-striatal pathway. The ventral tegmental area (VTA) works on the prefrontal cortex and the limbic system. The tuberoinfundibular pathway works with the caudate/putamen or striatum. There are actually two dopaminergic systems of interest when talking about TS. There is a chemical-physical and a chemical-emotional. The former is the nigro-striatal system and the latter the meso-cortical-limbic system. The nigro-striatal system operates an inhibitory action on movement. The substantia nigra inputs dopamine to the striatum and this in turn stimulates or activates movements.
No Dopamine results in rigidity and no spontaneity. The physical manifestation is tremors or Parkinsons. An excess of dopamine results in overactive, jerky & unwanted movements or TS. The meso-cortical-limbic system is involved with hyperactivity. The frontal and limbic play an important role in emotions. Abnormal levels of Dopamine in these pathways could result in Schizophrenia, TS, or a myriad of other disorders.
There are two motor areas. Effectively there are two systems. As outlined below, the latter is involved with TS. The cerebrum is involved with simple motor movements. The striatum, the substantia nigra and the globus pallidus are all involved in complex motor programs. As mentioned above. Low dopamine levels in these areas can result in Parkinson’s while high levels of dopamine can result in TS and schizophrenia. This is the area involved with TS.
4.2.3 Norepinephrine:
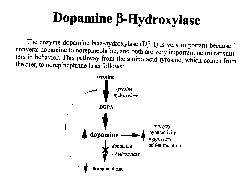
Norepinephrine
is a modulator of dopamine. Dopamine B - hydroxelase makes a slight change
to dopamine to make it norepinephrine. Norepinephrine is concentrated in the
area called the locus cerelus. Alpha 2 autoreceptors inhibit the release of
norepinephrine, while Beta autoreceptors stimulate release of norepinephrine.
There are two A2 receptors agonists: norepinephrine and clonidine. Studying
norepihephrine in isolation was too confusing; examining it in relation to
dopamine finally began to make sense. No norepinephrine means an increase
in dopamine. Norpeinephrine gets in the loop by acting as an agonist affecting
tyrosine hydroxelase.
4.2.4
Norepinephrine and Serotonin:
Norepinephrine acts as a tonic to serotonin nerves. Serotonin neurons from raphe nuclei have an inhibiting effect on norepineprine in the locus cerelus (70% of norepinephrine located here). A decrease in serotonin results in an increase in norepinephrine. Conversely an increase in serotonin results in a decrease in norepinephrine. Too little norepinephrine can cause depression but so can too little serotonin. Too much norepinephrine can cause mania. The serotonin and norepinephrine imbalance on their own can contribute to a myriad of behavioral disorders in the broader spectrum phenotype.
4.2.5 The Polygenic Inheritance of Behavior (Comings DE Search for the Tourette Syndrome and Human Behavior Genes 151):
The combinations based on the malfunctioning of the D2A1 receptor, the D beta-hydroxelase loop and the DAT 1 function serve each other in an additive and subtractive fashion.
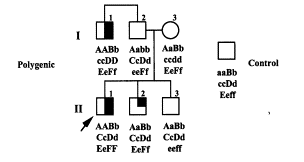
The inheritance model as illustrated above shows us a semi-dominant semi-recessive model. As far fetched as this model might be, it has been accepted by the Leckman et al study as a possible explanation for the expression of the behavior phenotypes of TS.
5.0 Mixed Models
5.1
Walkup, et al - 1996 - Mixed Model of Inheritance:
Walkup takes over from Pauls and Comings and identifies a mixed model of inheritance phenotype includes motor and phonic tics. Their results suggest that susceptibility for TS is conveyed by a major locus in combination with a multifactorial background.
The major locus accounts for over
half of the phenotypic variance for TS, whereas the multifactorial background
accounts for 40% of phenotypic variance. Penetrance estimates suggest that
all individuals homozygous for the susceptibility allele at the major locus
are affected, whereas only 2.2% of males and 0.3% of females heterozygous
at the major locus are affected. Of individuals affected with TS, 62% are
heterozygous and 38% are homozygous at the major locus. While none of the families had two
parents affected with TS, 19% of families had two parents affected with the
broader TS phenotype, which includes TS, chronic tic disorder, or obsessive
- compulsive disorder (Walkup, Family
Study 684).
Walkup has outlined his aims to include: 1. To confirm whether TS aggregates within families in a systematic way. 2. To identify whether the family data is most consistent with a mixed model, a single major locus model (dominant, recessive or additive), or a multifactorial model of inheritance. 3. To estimate gene frequency and penetrance rates. 4. To evaluate the relationship among TS, CT and OCD.
As outlined in the article, major locus accounts for over half of the phenotypic variance; multifactorial accounts for 40%. In terms of an inheritance model of individuals affected with TS, 62% are heterozygous and 30% are homozygous at the major locus. The gene frequency and penetrance estimates suggest that all individuals homozygous for the susceptibility allele at the major locus is affected; whereas, only 2.2% of males and 0.3% of females heterozygous at the major locus are affected. Lastly, while none of the families had two parents affected with TS, 19% of families had two parents affected with the broader TS phenotype, which includes: TS, chronic tic disorder, or obsessive-compulsive behavior.
Walkup’s genetic theory shifts away from a pure autosomal dominant theory to a mixed model. Walkup agrees with Comings (just like Leckman does) but does not include all the other behavioral manifestations identified by Comings. Walkup does however, move to a more multifactorial model (which includes an infectious side as an environmental feature) which is the prevalent model of the day. We are still miles away from Comings in that the possible related manifestations like alchoholism or eating disorders are still ignored.
Walkup’s significance lies in his treatment of exogenous factors in the etiology of TS. He includes a study of the impact of group A, B-hemolytic streptococcal infection or GABHS. The treatment and examination of GABHS is the subject of the infection section of this work.
Data in this study also support significant environmental contributions of TS, consistent with studies that have identified the association of non-genetic factors with tic symptom severity (Leckman et al. 1987, 1990; Hyde et al. 1992). Recent reports regarding the role of group A, B-hemolytic streptococcal infection in the development or exacerbation of TS and OCD suggest a role for additional non-genetic factors or a role for interaction of other genetically mediated vulnerabilities (Allen et al. 1995) (Walkup, Family Study 691).
Leckman, et al move to a more comprehensive multifactorial consideration of TS
The Leckman multifactorial model
is outlined in the below (Leckman 120):
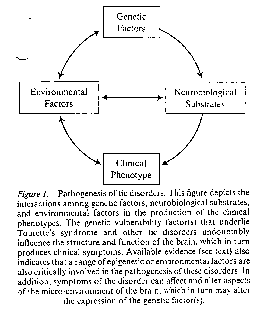
They champion the need for a standard TS phenotype. Who is going to define the standard?
Future progress requires the continued
use of well standardized diagnostic measures with demonstrated reliability
and validity (Leckman 122-3).
They cautiously ask that it be flexible. Who will make the additions?
Diagnostic concepts may need to
change in light of empirical data from genetic, neurobiological and treatment
studies (Leckman 122-3).
Many questions remain, they indicate, there is, according to Leckman, a lot of “opportunity for study.” It appears twin studies hold at least part of the puzzle from which this chaos will emerge a new phenotype standard.
Twin and family studies have also been used to establish the boundaries of the TS phenotype. There happens to be a consensus that chronic tic disorder and some OCD - should be included as alternate expressions of the TS phenotype (Leckman 122-3).
Comings has been attacked for being too broad. In reading Leckman, we have come full circle and we are back to autosomal dominant inheritance. In the same work, Leckman, states that even Walkup could not reject their model. They sound similar to Comings’ in their advocacy that more than one gene may be responsible for TS.
… possibility that the inherited
vulnerability to develop the disorder is due to several genes acting in concert
(Leckman 124).
And
… D2 Dopamine receptors, associated with tic severity (Leckman 122-3).
In their conclusion, they make rather profound statements concerning the definition, treatment and future of TS:
Although this knowledge is incomplete, efforts to integrate this information with clinical phenotypes has begun. The resulting dialectic offers the opportunity to generate testable hypotheses concerning many features of the clinical phenotype associated with Tourette’s syndrome by viewing them in light of our growing knowledge of the structural and functional constraints of these neural systems (Leckman 122-3).
6.0 Intervention / Drugs
Below is a cross listing (from the Tourette’s syndrome home page) of possible interventions that include at least one example from Comings’ broad-spectrum phenotype.
|
Motor and
Vocal Tics |
ADHD |
OCD |
Eating Disorders (Obesity) |
|
Haldol D2 Receptor blocker |
Ritalin Dopamine and Norepinephrine agonist |
Tofranil Noradrenergic reuptake inhibitor |
Prozac Serotonin and
Norepinephrine Reuptake Inhibitor |
|
Orad D2 Receptor blocker |
Dexedrine Dopamine and Norepinephrine agonist |
Anafronil Noradrenergic reuptake inhibitor |
Tofranil Noradrenergic reuptake inhibitor |
|
Mellaril Dopamine Receptor Antagonist |
Cylert Dopamine agonist |
Paxil Serotonin Reuptake Inhibitor |
Ritalin Dopamine and Norepinephrine
agonist |
|
Catapres Alpha 2 Norepinephrine agonist |
Tofranil Noradrenergic reuptake inhibitor |
Haldol D2 Receptor blocker |
Dexedrine Dopamine and Norepinephrine
agonist |
|
Risperdal Dopamine and Serotonine Modulator |
Norpramin Noradrenergic Reuptake Inhibitor |
Prozac Serotonin and Norepinephrine Reuptake Inhibitor |
Norparim Noradrenergic reuptake inhibitor |
An argument could be made that if one intervention works for two or more conditions, then a common underlying pathology may exist. Witness the use of Prozac for OCD as well as certain Eating Disorders. Also, take a hard look at the joint use of Haldol for Motor/Vocal Tics and OCD.
7.0 Conclusion
There are several conclusions one can draw from this examination of the genetic component of TS. First, that the chemical interaction of serotonin, dopamine and norepinephrine is complex. Second, that the evolution of a more complex mixed model is changing what is necessary and sufficient for TS. Third, intervention studies point to a linkage that suggest a common underlying pathology. Lastly, a unified approach to TS would certainly bolster a concerted approach to TS definition, prevalence and intervention.
The
chemical interaction of serotonin, dopamine and norepinephrine is complex.
Despite the complexity of the Serotonin - Dopamine Axis, there seems to be
an underlying assumption that the mechanism can be fully understood and calibrated
(Comings Search for the Tourette
Syndrome and Human Behavior Genes 95):
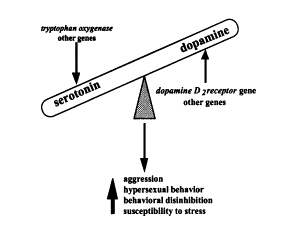
In section 4.2 of this study I undertook to examine the interrelation of serotonin, dopamine and norepinephrine. If the related studies are correct, a chemical imbalance can result in either a motor or behavioral disorder. This suggests that both motor and behavioral disorders might have a shared underlying pathology. In this light, a case for an inclusion of both motor and behavioral disorders can be made. More study, however, is required in this area.
The possibility of non-genetic infectious etiology complicates the simple genetic model. With results from studies done by Walkup, et al, and Leckman, et al shows that the evolution of a more complex mixed model is changing what is necessary and sufficient for TS. The impact of group A, B-hemolytic streptococcal infection or GABHS cannot be ignored.
The work of Dr. Louise Kiessling (personal communication) and her colleagues at Brown University clearly indicates that there is room for a serious examination of a non-genetic component to TS. Whether the pathology has a genetic or infectious origin, there seems to be a commonality between conditions that points to the chemical interaction of dopamine, serotonin and norepinephrine.
Arguments can be made back and forth concerning the addition and subtraction of particular phenotype expressions based on genetics linkage via pedigree studies or common underlying pathology via intervention studies. It is the core of this section not to offer an alternative phenotype, but to inspire the move to a more cooperative effort in a more systematic attempt at joint formal standardization of the Tourette’s syndrome phenotype.
Works Consulted
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 3rd Edition-Revised (DSM III R), Washington, D.C., American Psychiatric Association, 1987.
Bruun RD, Bruun B A Mind of Its Own Tourette’s syndrome: A story and a guide, 60-69 Oxford University Press, New York, 1994.
Comings DE Search for the Tourette Syndrome and Human Behavior Genes, Hope Press, Duarte, California, 1996.
Comings DE Tourette Syndrome and Human Behavior, Hope Press, Duarte, California, 1990.
Comings DE, Comings BG Alternative Hypothesis on the Inheritance of Tourette Syndrome, Advances in Neurology, Vol. 58, 189-199 edited by T.N. Chase, A.J. Friedhoff, and D.J. Cohen, Raven Press, Ltd., New York, 1992.
Compendium of Pharmaceuticals and Specialties, 3rd Edition, Ottawa, Ontario, Canadian Pharmaceutical Association, 1995.
Griffiths AJF, Miller JH, Suzuki DT, Lewontin RC, Gelbart WM, An Introduction to Genetic Analysis, 5th Edition, W.H. Freeman and Company, New York, 1993.
Kushner H, Kiessling LS The Controversy over the Classification of Gilles de la Tourette’s Syndrome 1800-1995 Perspective in Biology and Medicine, 39, 3 Spring 409-435, 1996.
Kushner H, Medical Fiction: The Case of the Cursing Marquis and the (Re)Construction of Gilles de la Tourette’s Syndrome Bull Hist Med, 69 224-254 1995.
Leckman JF, Peterson BS, Anderson GM, Arnsten AFT, Pauls DL, Cohen DJ Pathogenesis of Tourette’s Syndrome J Child Psychol Psychiat Vol. 38, No. 1, pp. 119-142, 1997.
McClearn GE, DeFries.JC Introduction to behavioral genetics, 133-158 W.H. Freeman and Company, San Francisco, 1973.
Pauls DL Issues in Genetic Linkage Studies of Tourette Syndrome: Phenotypic Spectrum and Genetic Model Parameters, Advances in Neurology, Vol. 58, 151-157, edited by T.N. Chase, A.J. Friedhoff, and D.J. Cohen, Raven Press, Ltd., New York, 1992.
Roberson MM, Mason A, Banerjee S, Eapen V, Zeitlin H The Prevalence of Gilles de la Tourettes Syndrome in a mainstream school, (under revue).
Walkup JT, LaBuda MC, Singer HS, et al. Family Study and Segregation Analysis of Tourette Syndrome: Evidence for a Mixed Model of Inheritance. Am J Hum Genet 59:684-693, 1996.
page
last updated 22 November 2004
Copyright © 2004 Miguel B. Llora, MA. All Rights Reserved.
Best viewed on Internet Explorer 5.x or later at a minimum of 1024 x 768 resolution